Due to its excellent casting properties, low density and good mechanical properties, sub-eutectic Al-Si alloys are widely used in aerospace and vehicle manufacturing fields, such as missile pylons, diesel engine bodies, cylinder heads, etc. Metamorphic eutectic Si is a commonly used method to improve the mechanical properties of Al-Si alloys. Antimony (Sb) is an effective metamorphite, and researchers are working to study the metamorphosis mechanism of SB. Studies by BIAN et al. have shown that Sb can promote eutectic Si nucleation. Wu Zhenping’s research shows that after the addition of Sb, the original nucleation core of eutectic Si, AlP, disappears, and AlSb becomes a new nucleation core. The research of HANSEN et al. shows that the appearance of AlSb is the result of Sb’s over-metamorphism of eutectic Si. Qi Xiaogang et al. have shown that Mg3Sb2 can become the nucleation core of eutectic Si. RESEARCH BY YANEVA ET AL. AND KHAN ET AL. SHOWS THAT THE MECHANISM OF SB METAMORPHIC EUTECTIC SI, IS THAT SB IS ADSORBED ON THE SURFACE OF EUTECTIC SI, HINDERING ITS GROWTH. In conclusion, although many scholars have studied the mechanism of Sb metamorphic eutectic Si, their views are different, and the mechanism of Sb metamorphic eutectic Si needs further study. Due to the low cost, simple process and short cycle, gravity sand casting
It is indispensable in the foundry industry. Subeutectic Al-7Si gold group
The weaving includes primary α-Al grains, eutectic Si, nanoprecipitated phases, and impurities
Appearance. However, under the casting conditions of heavy sand mold, there are few studies on the influence of Sb on the primary α-Al grain and eutectic Si of sub-eutectic Al-7Si alloy, so this paper conducts related research, hoping to provide some reference for the industrial application of Sb in sub-eutectic Al-Si alloy and the modification and upgrading of Al-7Si alloy.
1Experimental materials and methods
Melt and stir evenly the refined aluminum, Al-12Si and Al-4Sb in the furnace, add Mg when the temperature is controlled to 710~720 °C, stir for 3~5min, and pour the sample after refining. Direct reading spectroscopy was used to determine the actual formation of the alloy
The results are shown in Table 1. Take advantage of FLUKE2638A data mining
The collector and type K thermocouple take a temperature-time curve of the solidification, each
The curves are collected three times. Samples were prepared under 25V voltage, 1% fluoroborate medium anodic coating for 5.5min, and the nascent α-Al grains were observed by BX53MRF-S polarizing microscope. In order to observe eutectic Si more clearly, the sample was soaked in 20% NaOH solution for 5min to remove the Al matrix around eutectic Si, and the TESCAN MIRALMS scanning electron microscope was used to observe eutectic Si. The nucleation core of eutectic Si was observed by electron backscatter diffraction, and the sample was thinned by ion milling. Using the random wire cutting method to calculate the crystal size, the length and width of eutectic Si were counted using Image-ProPlus software, counting more than 40 grains and 120 eutectic Si. FEITecnaiF20 transmission electron microscope was used to observe the twins of eutectic Si, and the samples were also thinned by ion milling; The electron microscope is used to analyze the chemical composition of eutectic Si with an energy spectrometer.
Table 1 Chemical composition of Al-Si alloy
| 编号 | Si | Mg | Ti | Fe | B | Sb | Al |
| 1# | 6.80 | 0.48 | 0.12 | <0.05 | <0.05 | 0 | 余量 |
| 2# | 6.90 | 0.50 | 0.14 | <0.05 | <0.05 | 0.09 | 余量 |
2 Results and discussion
2.1 Effect of Sb on nascent α-Al grains
Figure 1 shows the temperature-time curves of solidification of alloys 1# and 2#, TAlN
is the nucleation temperature of the primary α-Al grain, and TSiN is the starting temperature of the eutectic reaction, that is, the eutectic Si nucleation temperature. It is known from Figure 1(a) that without the addition of Sb, the TAlN of Al-7Si alloy is 619.5 °C and TSiN is 578.1 °C. It can be seen from Figure 1(b) that with the addition of 0.09% Sb, the TAlN of Al-7Si decreased to 611.8 °C and TSiN decreased to 572.6 °C.
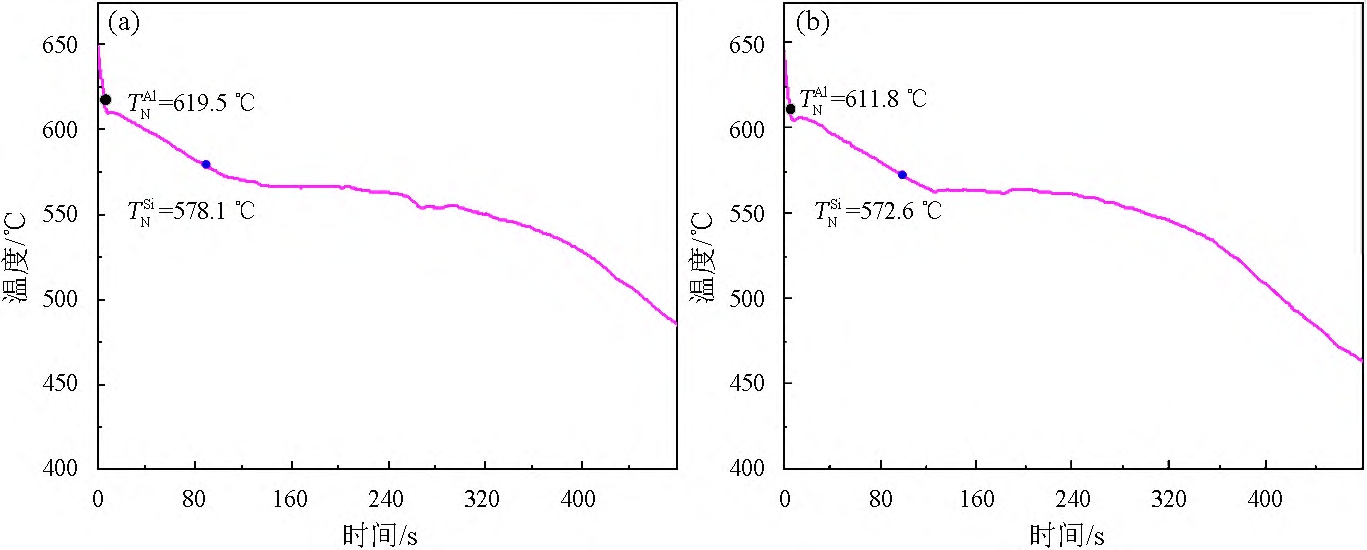
Figure 1 Temperature-time curve of solidification process of Al-7Si alloys of 1# (a) and 2# (b).
Figure 2 shows an image of the nascent α-Al grain of Al-7Si gold.
Figure 2(a) shows that when Sb is not added, most of the primary α-Al grains of Al-7Si alloy are dendrite crystals, a few are equiaxed crystals, and the grains are average
The size is 319 μm. Figure 2(b) shows that after the addition of 0.09% Sb, the morphology of the primary α-Al grain of Al-7Si alloy did not change much, but the average grain size increased to 353 μm. A primary α-Al grain nucleus is required
The lower the actual nucleation temperature, the greater the required supercooling during nucleation, the more difficult the grain nucleation is. The TSiN on the solidification temperature-time curve in Figure 1 is the actual nucleation temperature of the nascent α-Al grain. It can be seen from Figure 1 that after adding 0.09% Sb, the actual nucleation temperature of the primary α-Al grain of Al-7Si alloy decreases, indicating that the required subcooling of the nucleation increases, and the grain nucleation becomes difficult, so the grain size increases.
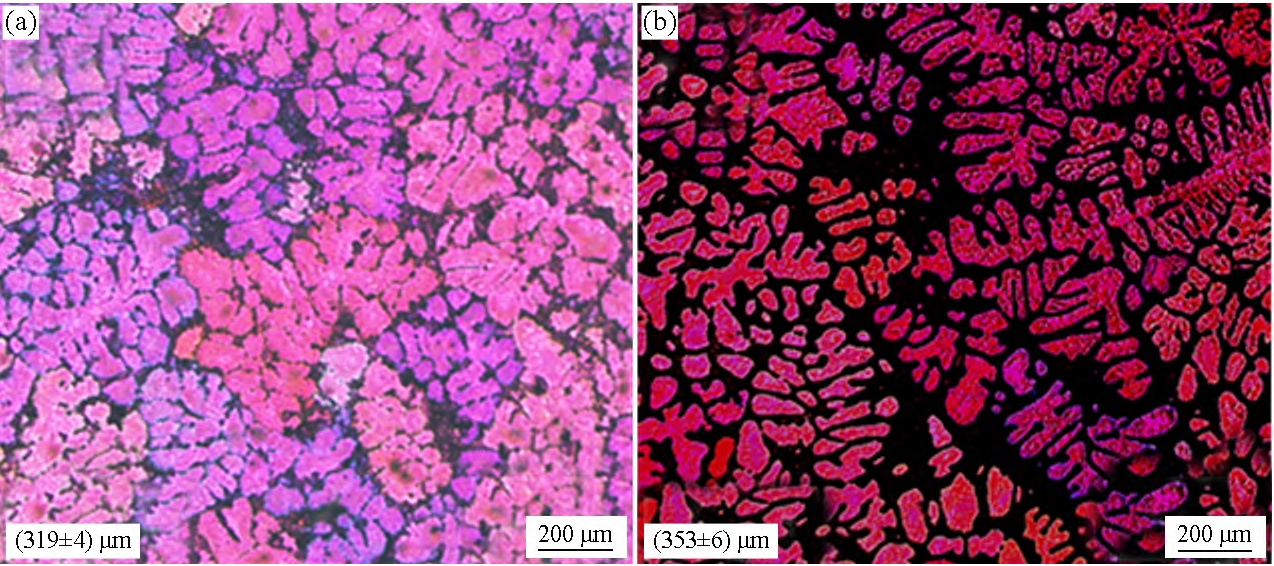
Figure 2 Polarizing microscopy image of primary α-Al grains in Al-7Si alloys 1# (a) and 2# (b).
2.2 Effect of Sb on eutectic Si
Figure 3 shows the morphology, length and width of eutectic Si of Al-7Si alloy. As can be seen from Figure 3(a), when Sb is not added, the eutectic Si of Al-7Si alloy is plate-like, with an average length of 19.7 μm and an average width of 8.1 μm. As can be seen from Figure 3(b), after adding 0.09% Sb, the eutectic Si of Al-7Si alloy becomes shorter and narrower, the average length is reduced to 13.3 μm, and the average width is reduced to 5.4 μm. It can be seen that Sb has a good metamorphic effect on the eutectic Si of Al-7Si alloy. According to the phase diagram of the Al-Si binary alloy, the lower the temperature, the smaller the solid solubility of Si in α-Al. During the solidification process, the driving force of Si atoms leaving α-Al is the chemical potential of Si atoms, and the greater the chemical potential, the faster the Si protons detach, and this chemical potential is the difference between the content of Si atoms and the solid solubility. As can be seen from Figure 1, after adding Sb, the eutectic Si nucleation temperature TSi
N decreases, at this time the solid solubility of Si in α-Al decreases, but the content of Si atoms in the experimental design is the same, that is, the chemical potential increases, the speed at which Si protons are squeezed into the front of the solid-liquid interface increases, and the growth rate of eutectic Si increases
fast, while the growth rate of eutectic Al accelerates, resulting in a shorter and narrower eutectic Si.
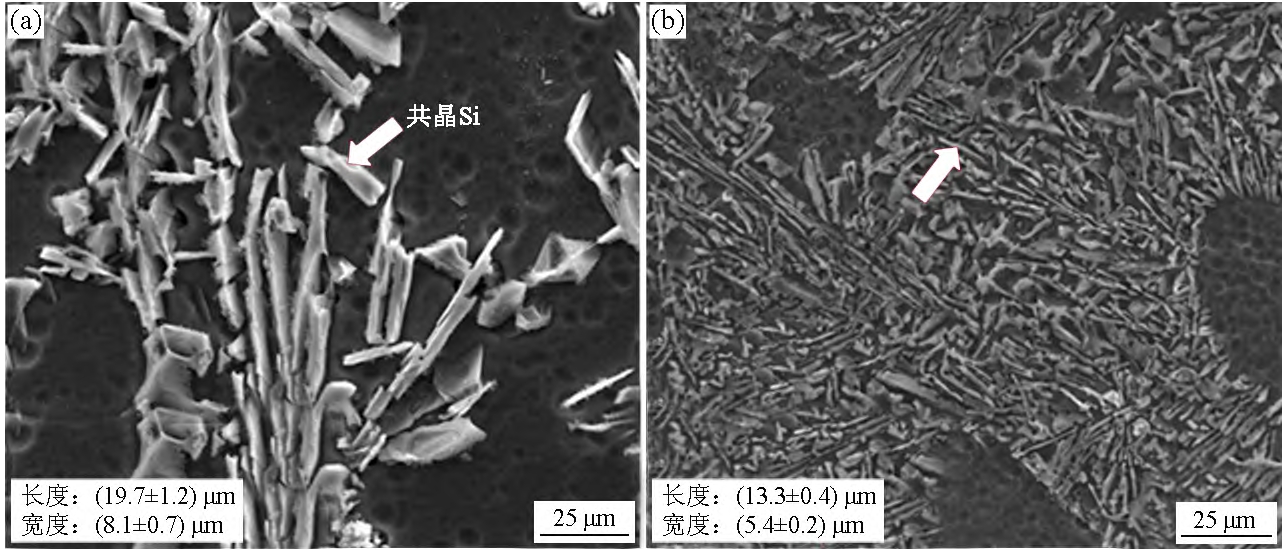
2.3 Mechanism of Sb metamorphic eutectic Si
The metamorphosis of eutectic Si is closely related to its nucleation core and number of twins. Figure 4 is an image of the nucleated core of eutectic Si observed using EBSD, with arrows identifying eutectic Si. If total
Crystal Si is born from the same nucleus core, and the orientation is the same, that is
EBSD images are identical; If the nucleus of the EBSD is different, the color on the EBSD photograph is different, that is, the more colors of the eutectic Si region on the EBSD image, the more nucleation cores indicating the eutectic Si. As can be seen from Figure 4, after adding Sb, the color variety of eutectic Si region on the EBSD image does not change much, which indicates that Sb does not increase the number of eutectic Si nucleated cores under gravity sand casting conditions
to metamorphize eutectic Si. Figure 5 is a TEM image of alloy 2#. As can be seen from Figure 5(a), after adding Sb, no twins appear in eutectic Si. The energy spectrum shown in Figure 5(b) indicates that the element Sb is not present in eutectic Si (Cu atoms are derived from the Cu network supporting the sample). No characteristic spots of twins are observed in Figure 5(c), indicating that no twins have formed in eutectic Si. If the spoilage agent passes
If the method acting on the twin metamorphizes eutectic Si, there will be atomic enrichment of the metamorphizer at the twin of the eutectic Si. It can be seen that under the gravity sand casting condition, Sb does not metamorphize eutectic Si by acting on the twins. If the metamorphizer atom can induce eutectic Si to produce twinning, the diameter ratio of the metamorphizer atom to Si atom is close to 1.65, compared to Sb
The diameter ratio of atoms to Si atoms is 1.32, so Sb is added
Later, no more twins appeared in eutectic Si. In summary, it can be seen that Sb does not act metamorphically by acting on the nucleation core and twin of eutectic Si.
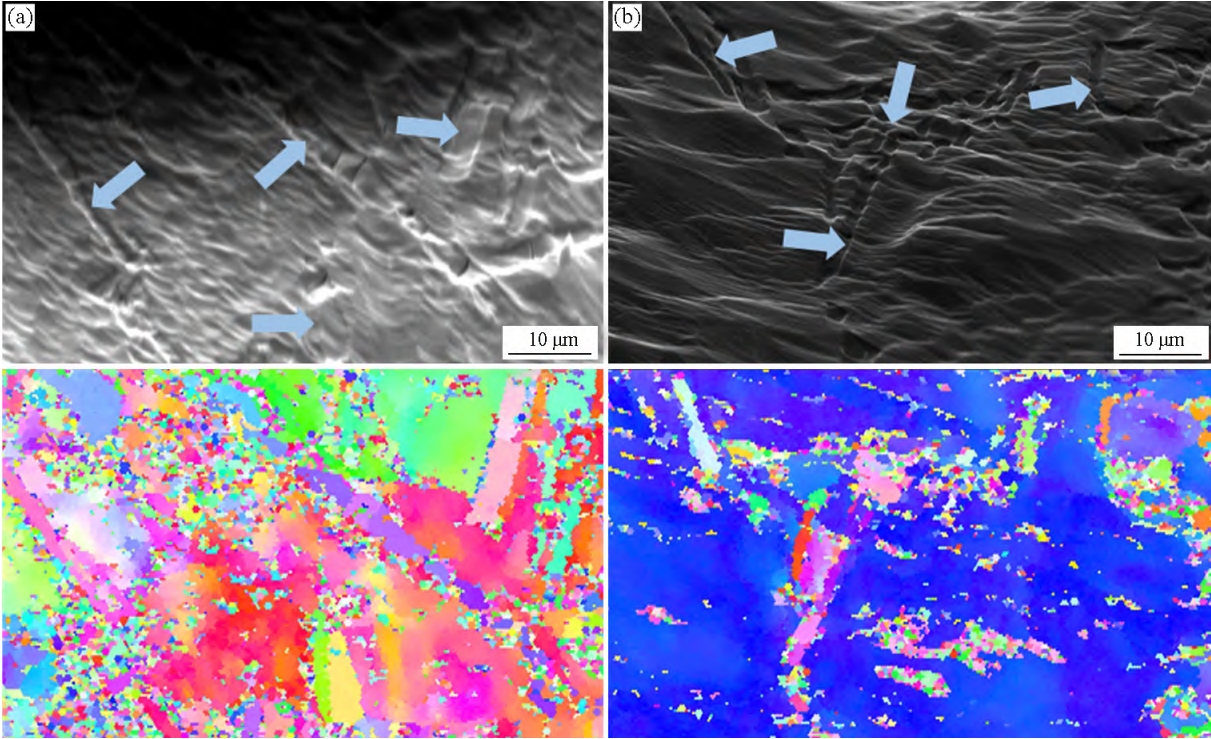
Figure 4 EBSD image of the co-crystalline Si-nucleated core of Al-7Si alloys 1# (a) and 2# (b).
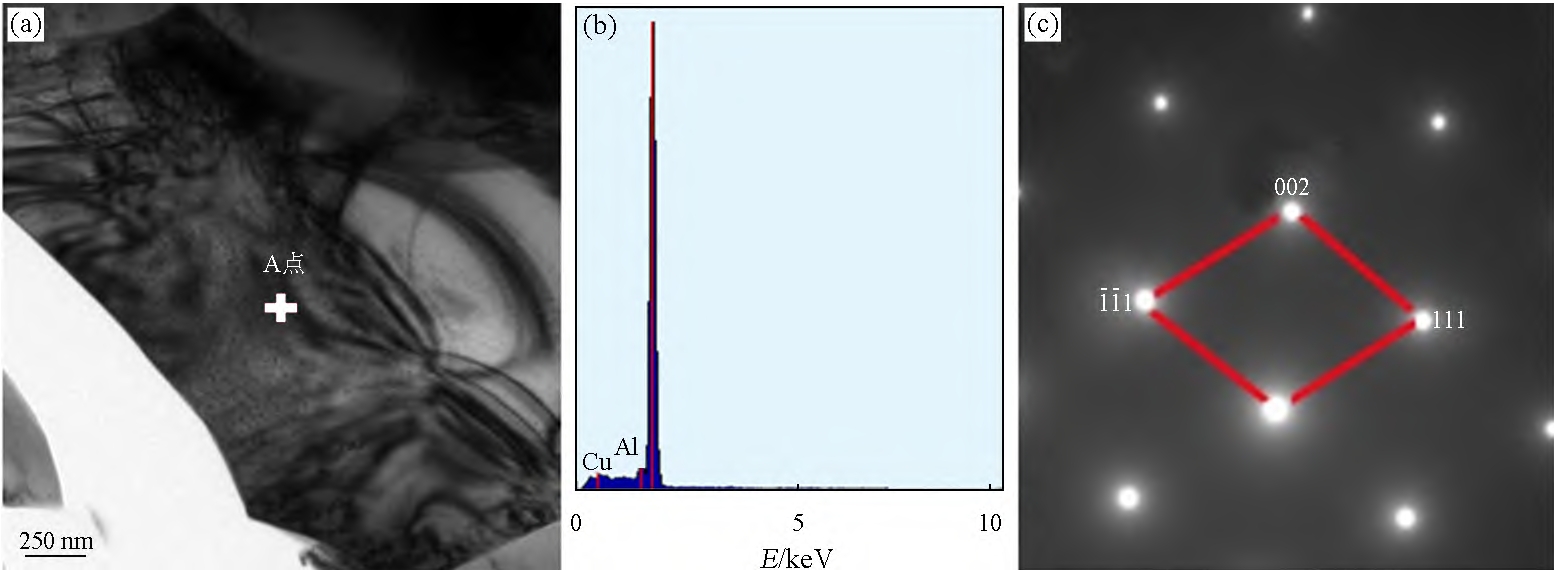
Figure 5 TEM analysis results of 2# alloy
3 Conclusion
1) After adding Sb, the heavy sand mold casts the initial stage of Al-7Si gold
The nucleation temperature of α-Al grains and eutectic Si decreased. beginning
α-Al grain nucleation is more difficult, and the average particle size increases from 319 μm to 353 μm. 2) Sb in gravity sand casting The mechanism of metamorphic eutectic Si in Al-7Si alloy is to reduce the nucleation temperature of the primary α-Al grain, and Si is in
The solid solubility in α-Al decreases, the chemical potential increases, the rate of expulsion of Si protons from α-Al increases, the growth rate of eutectic Si increases, and finally eutectic Si becomes shorter and narrower. Sb does not act on the nucleus of eutectic Si
Metamorphic effects on the core and twin.
